Electron Configuration for Nitrogen (N and N3 ion)
The electron configuration of an element is the arrangement of its electrons in its atomic orbitals. By knowing the electron configuration of an element, we can predict and explain a great deal of its chemistry.. The electron configuration of nitrogen is thus 1s 2 2s 2 2p 3. At oxygen, with Z = 8 and eight electrons, we have no choice. One.

Nitrogen Protons Neutrons Electrons Electron Configuration
The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. We describe an electron configuration with a symbol that contains three pieces of information ( Figure 6.25 ): The number of the principal quantum shell, n,
Electron Configuration (Elements 120) Good Science
The arrangement of electrons in nitrogen in specific rules in different orbits and orbitals is called the electron configuration of nitrogen. The electron configuration of nitrogen is [ He] 2s 2 2p 3 , if the electron arrangement is through orbitals. Electron configuration can be done in two ways.

How to find Valency? What are valence electrons? Teachoo
Electron configuration chart of all Elements is mentioned in the table below. The Shorthand electron configuration (or Noble gas configuration) as well as Full electron configuration is also mentioned in the table. Free Gift for you: Interactive Periodic Table Let me tell you how this Interactive Periodic Table will help you in your studies. 1).

Diagram representation of the element nitrogen Vector Image
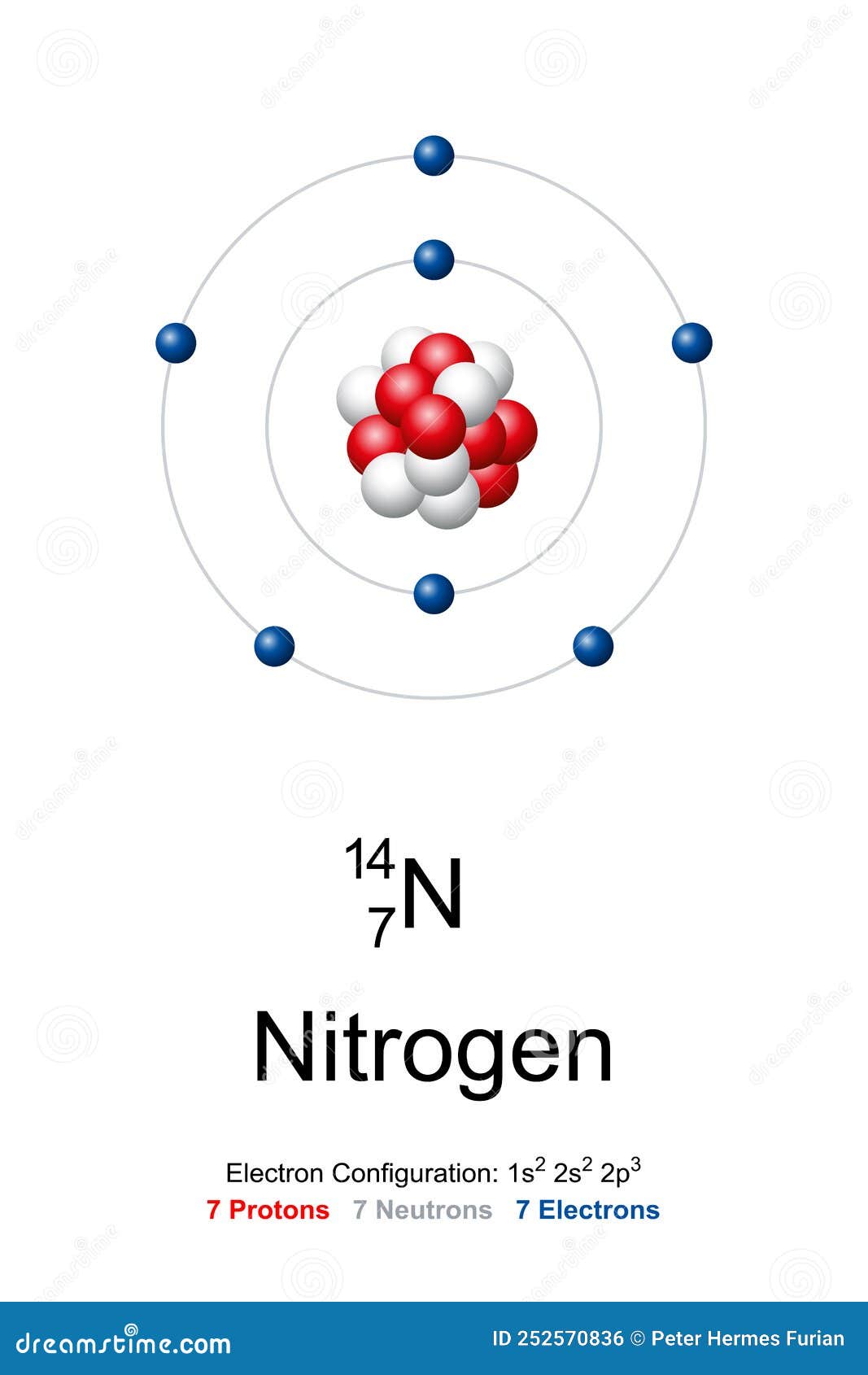
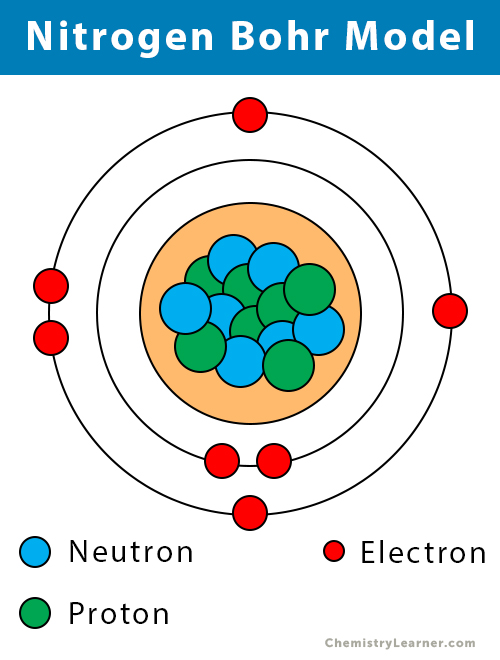
Nitrogen-16 is composed of 7 protons, 9 neutrons, and 7 electrons. In nuclear reactors, nitrogen-16 can be used to detect leakages from steam generators. Nitrogen-16 is an isotope of nitrogen generated by neutron activation of oxygen contained in the water. It has a short half-life of 7.1 sec and it decays via beta decay.

Orbital Diagram For Nitrogen (N) Nitrogen Electron Configuration
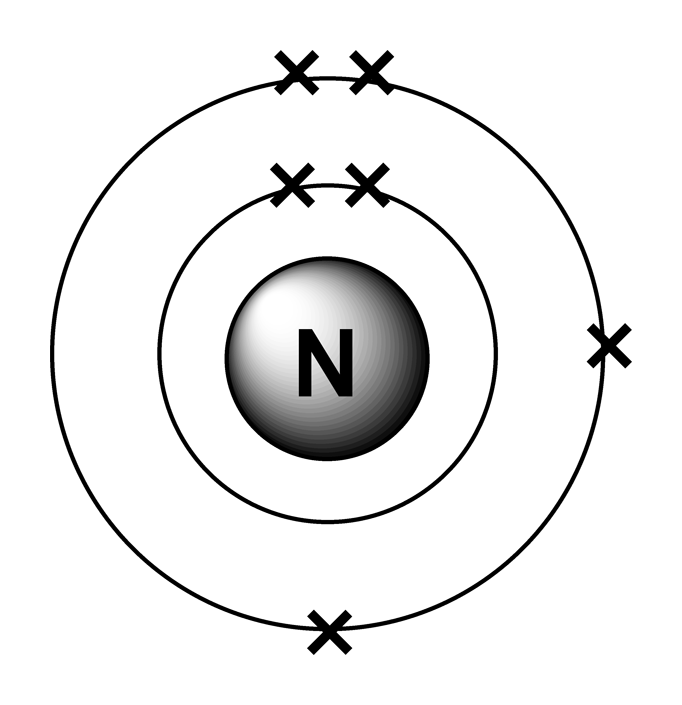
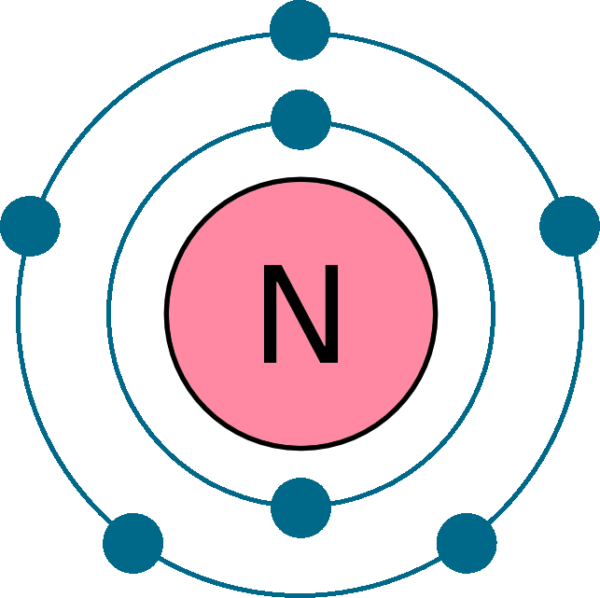
An electron configuration diagram is a model that depicts the position of electrons as they orbit the nucleus of an atom. Electrons are represented by dots or crosses and are positioned in energy levels, or 'shells', around the central nucleus. This is sometimes called the Bohr, or the 'solar system', model. Download this
Nitrogen Electron Configuration (N) with Orbital Diagram
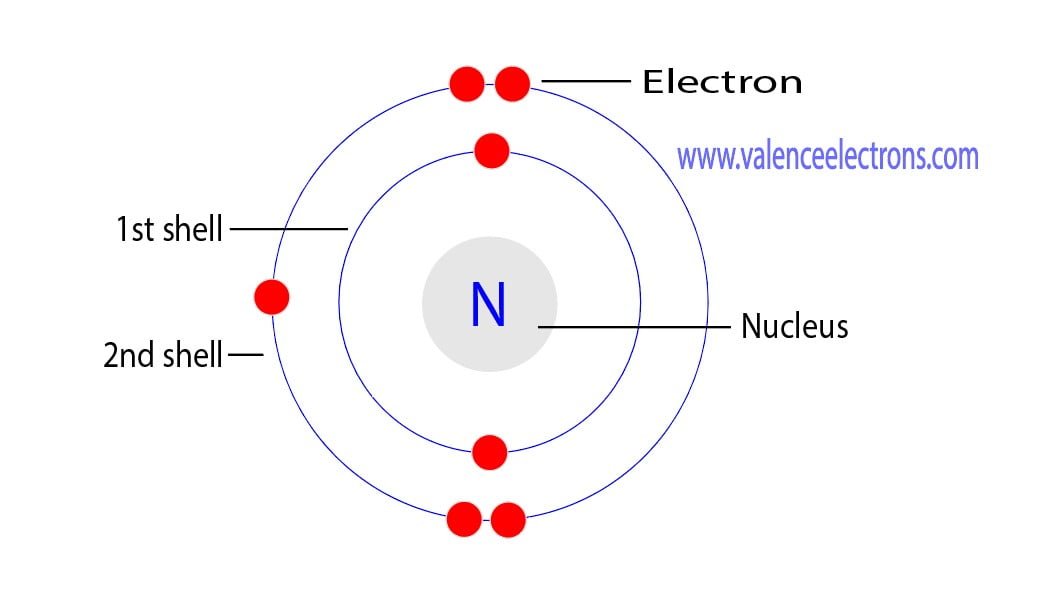
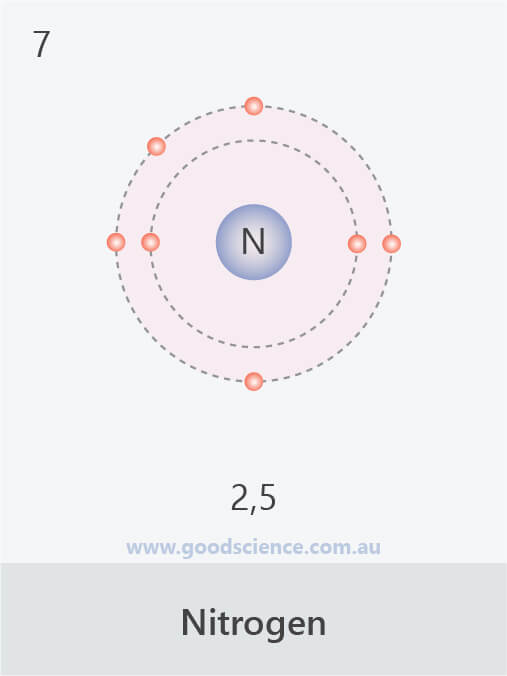
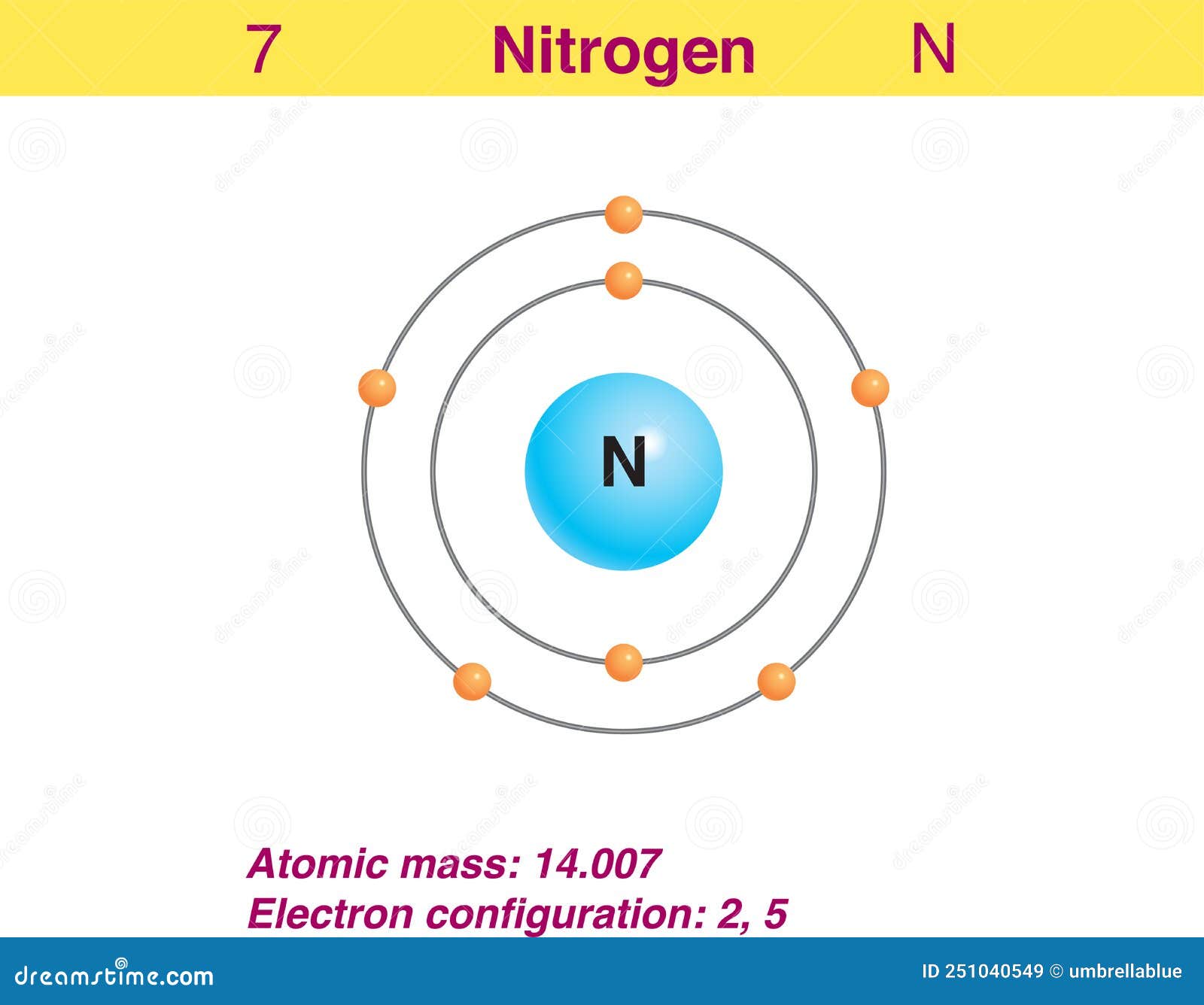
This electron arrangement indicates that the outermost orbit of Nitrogen (N) has 5 electrons. Hence, it lies in group 15. Why is Nitrogen in Period 2? Let me ask you a question. How many shells does Nitrogen have? It's 2. Right? You have already seen the bohr model of nitrogen element in the above table.
Electron arrangements
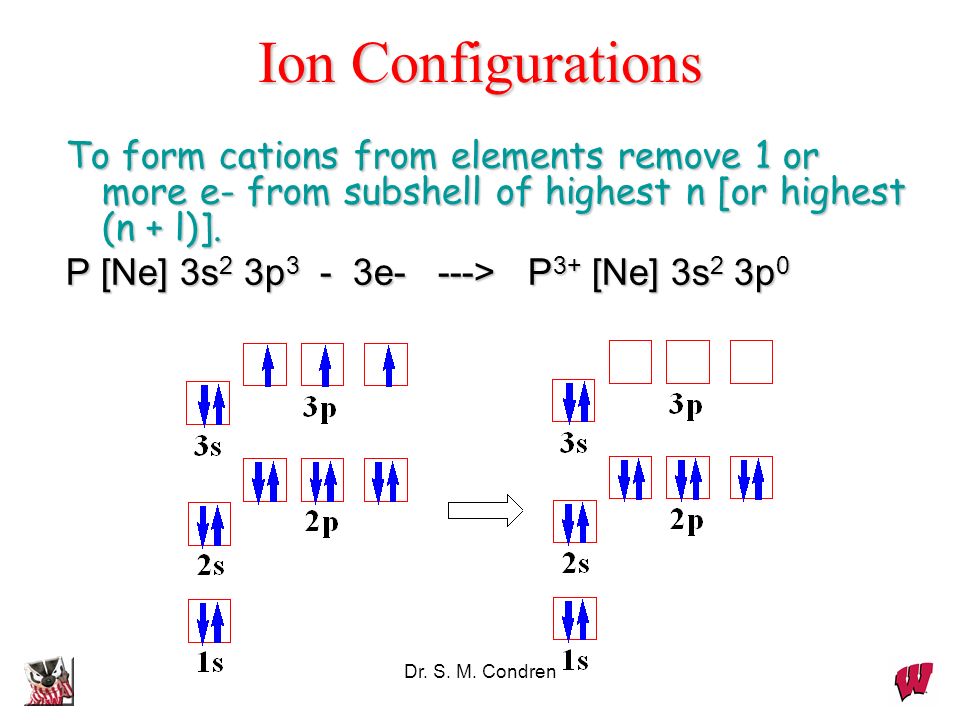
The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to represent an atom that has ionized into a cation or anion by compensating with the loss of or gain of electrons in their.
How many valence electrons does nitrogen have? Ask4Essay
Answers. Electrons are organized into shells and subshells around nuclei. The electron configuration states the arrangement of electrons in shells and subshells. Valence electrons are in the highest-numbered shell; all other electrons are core electrons.
Nitrogen, Atom Model of Nitrogen14 with 7 Protons, 7 Neutrons and 7 Electrons Stock Vector
Arrangement of Electrons Energy Levels or Shells The simplest model of electrons has them orbiting in shells around the nucleus. Each successive shell is further from the nucleus and has a greater energy. Sub Shells and Orbitals This model can be further refined by the concept of sub shells and orbitals.

How to write the Electronic Configuration of Nitrogen Chemical Bonding YouTube YouTube
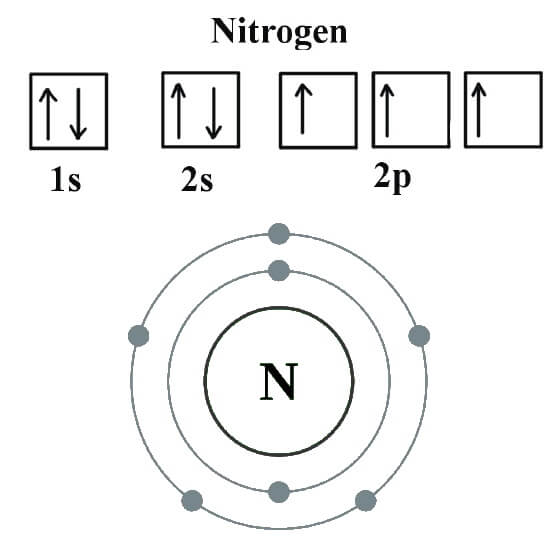
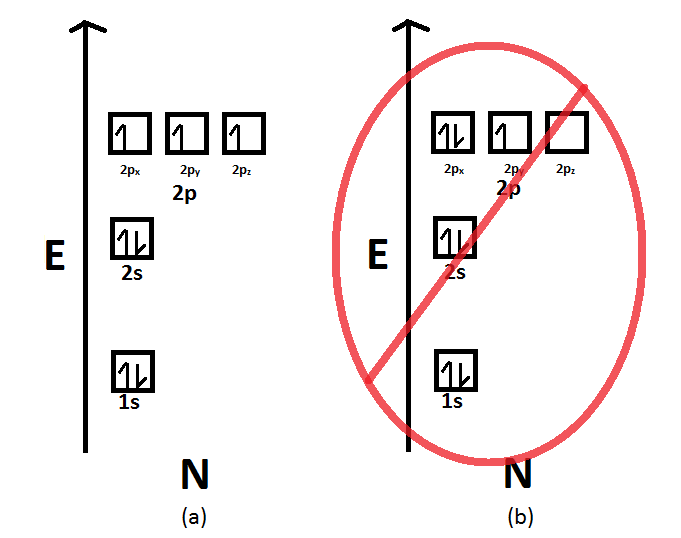
Nitrogen is the seventh element of the periodic table with a total of 7 electrons. When we write the electron configuration of N the first two electrons go in the 1s orbital. As 1s can only hold 2 electrons and the other next two electrons for Nitrogen (N) go in the 2s orbital. The three electrons that are remained will go in the 2p orbital.
Nitrogen Facts, Symbol, Discovery, Properties, Uses
The electron configuration of nitrogen is written by following steps: Nitrogen with atomic number 7, has 7 electrons, which is significant to write electron configuration. Considering the n+1 energy rule, the electron configuration is written based on Aufbau principle.
Electron of the Element Nitrogen Stock Vector Illustration of print, atom 251040549
Nitrogen is the seventh element with a total of 7 electrons. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for N goes in the 2s orbital. The remaining three electrons will go in the 2p orbital.

What is the number of valence electrons in nitrogen? What's Insight
1 to 20: Predicting an electron arrangement The electron arrangement of an atom can be predicted from its atomic number. For example, the atomic number of sodium is 11. Sodium atoms have 11.
Electron Configuration Chemistry LibreTexts
For example, the ground state electron configuration of nitrogen (1 s 2 2 s 2 2 p 3 \rm 1s^22s^22p^3 1 s 2 2 s 2 2 p 3) indicates that it has 3 3 3 electrons occupying the 2 p 2 \rm p 2 p orbital.. This is such a stable electron shell arrangement that they essentially have no valence electrons available for bonding or reacting.
Nitrogen Element With Reaction, Properties, Uses, & Price Periodic Table
Locations & contacts Element Nitrogen (N), Group 15, Atomic Number 7, p-block, Mass 14.007. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images.